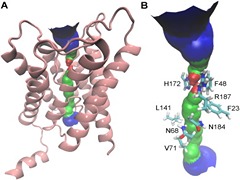
Formate channels play a crucial role in the metabolism of several different kinds of bacteria by bringing about uptake and export of formate ions. The current study investigates the structure, dynamics and ion channel activity of the formate channel FocA from Escherichia coli by employing extensive molecular dynamics (MD) simulations. The channel, known to be pH-sensitive, is modelled under different pH conditions by considering two different protonation states of a histidine residue. The results show that a fall in pH brings about an enhancement of the formate-conducting activity of the channel. The increased conductivity is a consequence of an overall widening of the pore at low pH, with the widening being brought about by movements of a pore-facing helical domain and a loop region.
pH-mediated gating and formate transport mechanism in the Escherichia coli formate channel

S Padhi, LK Reddy, UD Priyakumar,